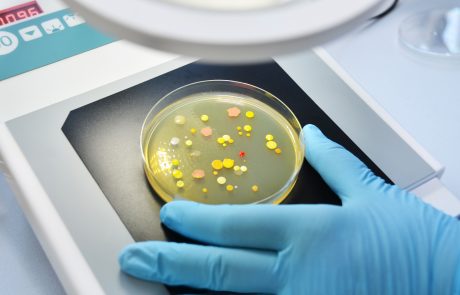
Welcome to our Lab
The Microbiological Test Laboratory Bad Elster is a testing laboratory for medical devices accredited by DAkkS (national accreditation body of Federal Republic of Germany) according to DIN EN ISO/IEC 17025. It is licensed for performing work governed by the provisions of §144 IfSG (German Infection Protection Act).
Our quality management enables transparent control and monitoring of all work procedures as well as flexible planning, and ensures the high quality of the test results.
All work is performed according to current regulations, and is based in particular on the DIN, EN and ISO standards as well as the European Pharmcopoeia.
Our facility is equipped with modern laboratory rooms with controlled clean room conditions. Our technical equipment is state-of-the-art.
As a small, highly qualified team, we process assignments Europe-wide, customized to your requirements, with personal attention and at short notice.
Our Team
Managing Director
Dr. rer. nat. Kathrin Stark
stark@mtl-be.de
Head of Laboratory
Dipl-Biol. Yvonne Dressel
dressel@mtl-be.de
Office
info@mtl-be.de
Our Lab
Services
Testing of medical devices
+ Determination of the population of microorganisms on products (bioburden) in accordance with DIN EN ISO 11737-1
+ Test of sterility according to DIN EN ISO 11737-2
Tests for sterility are performed in accordance with the EC Guide to Good Manufacturing Practice and Ph. Eur. under a Class A biological safety cabinet in a Class B clean room.
+ Bacterial endotoxins test (BET) according to Ph. Eur.
Monitoring of cleanrooms and associated controlled environments
+ Determination of the airborne viable microorganism count according to DIN EN 17141
- Airborne microorganism measurement using active air samplers
- Determination of airborne colony count using settle plates
+ Determination of the airborne particle concentration acc. to DIN EN ISO 14644
+ Detection of microbial contamination of surfaces acc. to DIN EN 17141
+ Personnel hygiene
+ Microbiological testing of purified water
Monitoring of hospital hygiene
+ Hygiene testing of ventilation systems in rooms of health care
- Testing for airborne microorganism count
- Testing for airborne particle count
+ Determination of the colony count on surfaces
- Personnel hygiene
- Surface disinfection testing
+ Testing of sterilizers and sterilization processes using bioindicators
+ Testing of cleaning and disinfection processes using bioindicators
+ Microbiological testing of purified water (DI water)
Quality assurance for reprocessing of medical devices
Cleaning, disinfection and sterilization of medical devices is, in accordance with legal requirements, to be performed using validated procedures in such a way that the success of these procedures is demonstrably ensured and the safety and health of patients, users and third-party persons is not endangered (German Medical Devices Act, Medical Devices Operator Ordinance)
+Performance evaluation within the scope of validation of sterilization processes (DIN EN ISO 17665-1)
- with moist heat (steam sterilizaion)
- with low-temperature steam/formaldehyde
+ Performance evaluation within the scope of validation of cleaning and disinfection processes in mechanical reprocessing of medical devices
Biological indicators for sterilization processes
Moist heat sterilization – Sporotest-D
Dry heat sterilization – Steri-Test-H
Biological indicators for disinfection and decontamination
Washer disinfectors (WD) – Desi-Test-R
Human waste containers– Desi-Test-S
Bedframes, bedside tables– Desi-Test-B
Laundry disinfection – Desi-Test-W
(S. aureus or cfu 10^7 for request)
Surgical shoes– Desi-Test-Shoes
Dishwashers – Desi-Test-G
Multitank-transport dishwashers – MTG-Test
Verification the reprocessing of thermolabile endoscopes
Ordering forms
Download the required forms to your computer, print them out and send them back to us by fax or email.
Fax-Nummer: +49 (0)37437 2418 email: info@mtl-be.de
Testing of medical devices
• Form testing medical device.pdf
Hygienic environmental monitoring
• Form monitoring.pdf
Contact
Mikrobiologisches Testlabor GmbH Bad Elster
(Microbiological Test Laboratory)
Brambacher Straße 17
08645 Bad Elster / OT Mühlhausen
Germany
Phone: +49 (0)37437 2208
Fax: +49 (0)37437 2418
E-Mail: info@mtl-be.de

Access route
Motorway A72 or A9 via connection point Bayerisches Vogtland until exit Plauen Süd, Bundesstraße (Federal Highway) B92 via Oelsnitz to Bad Elster – local district Mühlhausen (direction of Bad Brambach)
Legal notice
Address
Mikrobiologisches Testlabor GmbH Bad Elster
Brambacher Straße 17
08645 Bad Elster – OT Mühlhausen
Phone: +49 (0)37437 2208
Fax: +49 (0)37437 2418
E-Mail: info@mtl-be.de
Managing Director
Dr. rer. nat. Kathrin Stark
Registration court: Chemnitz Municipal Court
Registration number: HRB 18349
Tax number: DE 211568713
Responsible for the contents pursuant to § 6 MDStV (German Media Tax ordinance): Dr. Kathrin Stark
Privacy
The Microbiological Test Laboratory Bad Elster takes the protection of your personal data very seriously and adheres strictly to the rules of data protection law. Personal data is only collected to the extent necessary for this web application. Under no circumstances will the data collected be sold or passed on to third parties for any other reason. The data collected will only be processed for the stated purpose.
The following declaration gives you an overview of how we guarantee this protection and what type of data is collected and for what purpose.
Read moreCALL
+ 49 (0) 37437 2208
E-MAIL
info@mtl-be.de
ADDRESS
MTL GmbH Bad Elster
Brambacher Straße 17
08645 Bad Elster
– OT Mühlhausen –